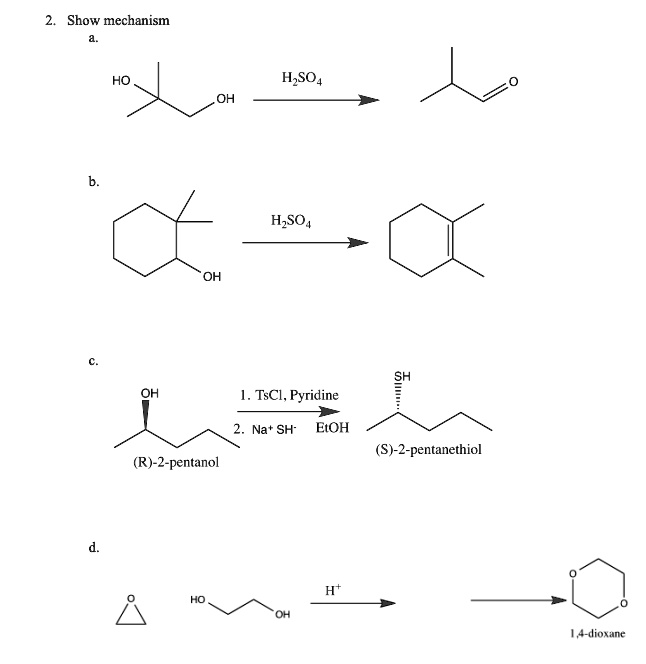
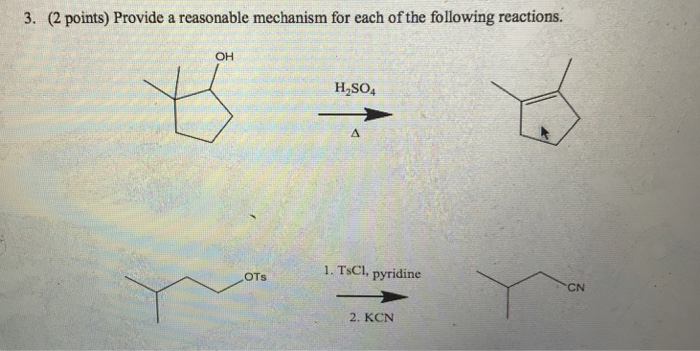
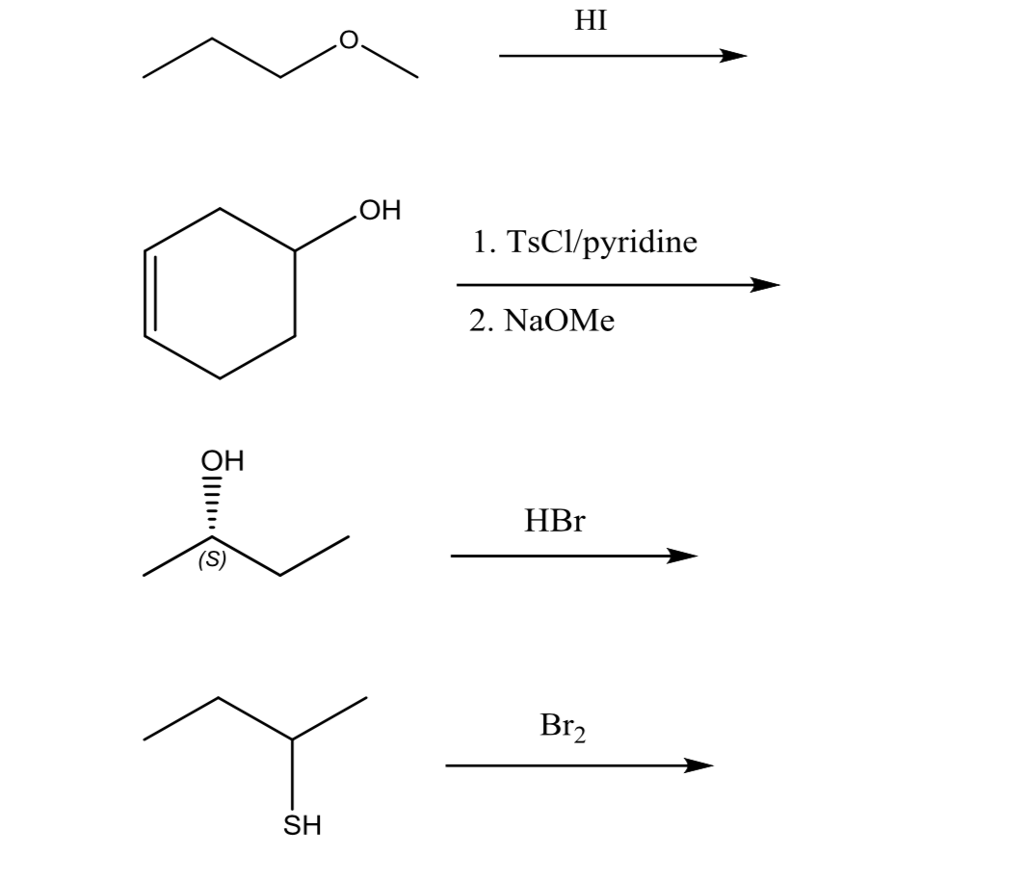
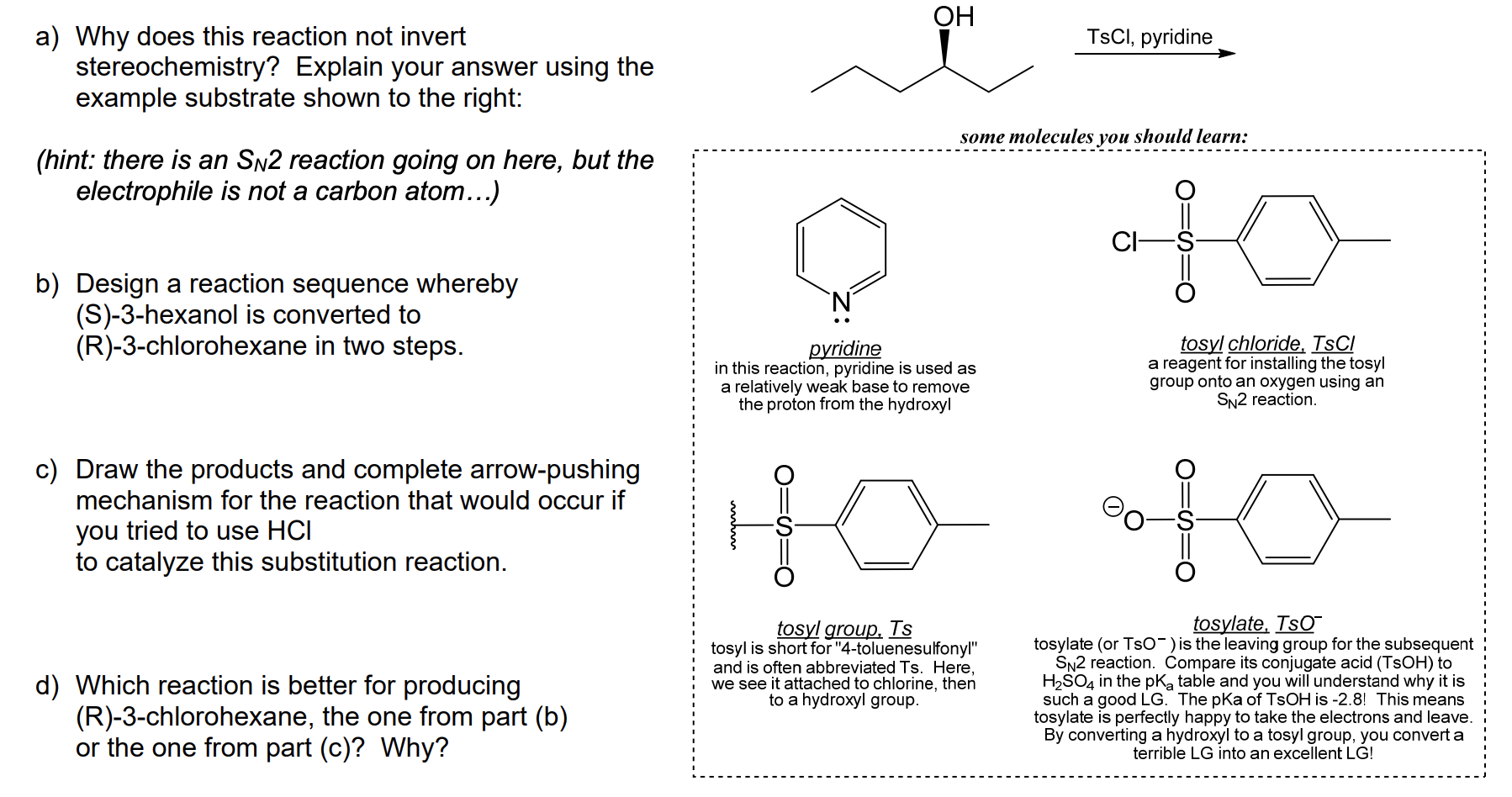
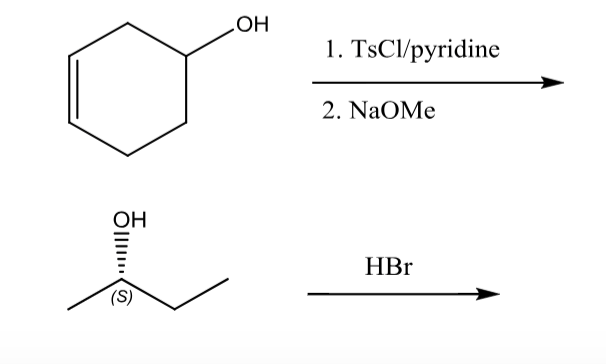
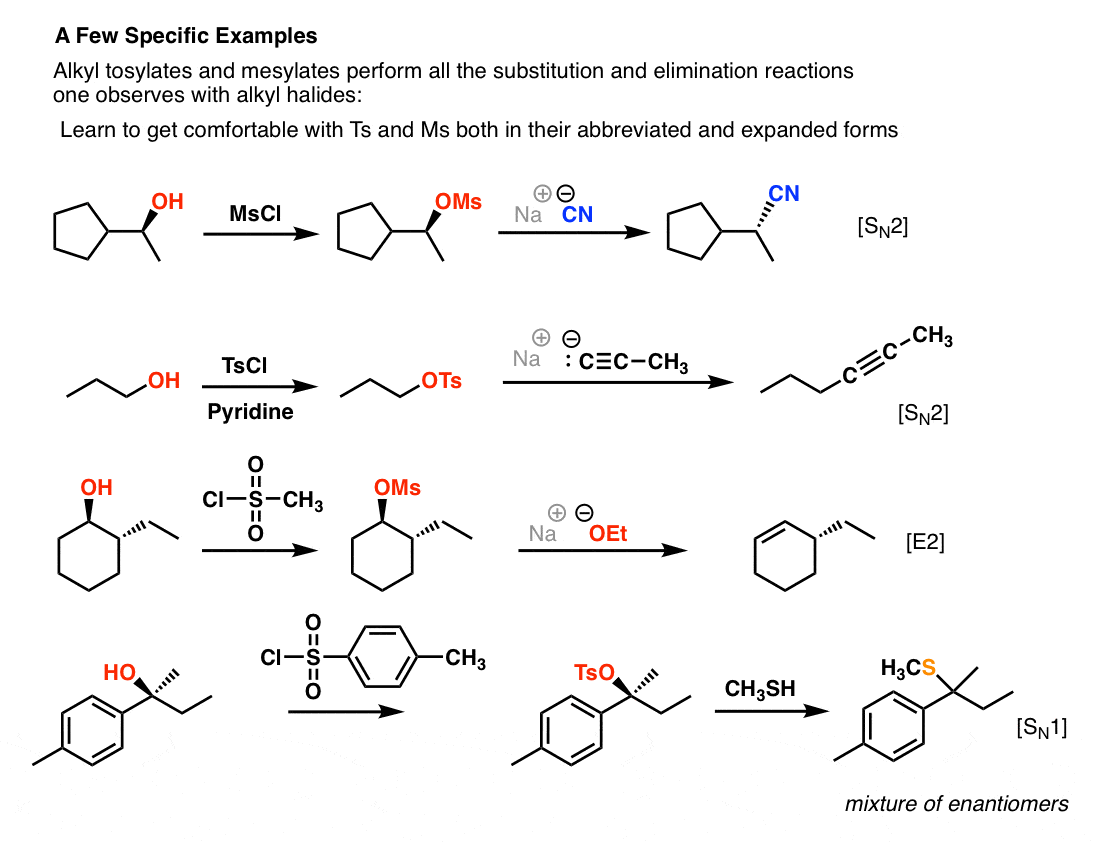
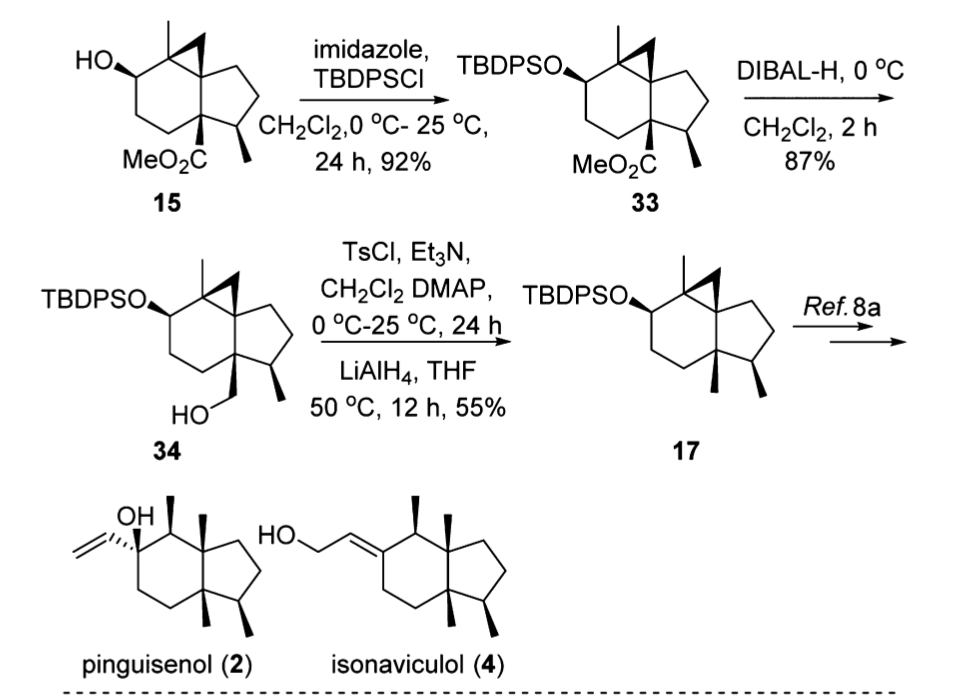
Reactions of Alcohols Oxidation R-X, Ether, and Ester Preparation Protection of Alcohols Synthesis The Logic of Mechanisms. - ppt download
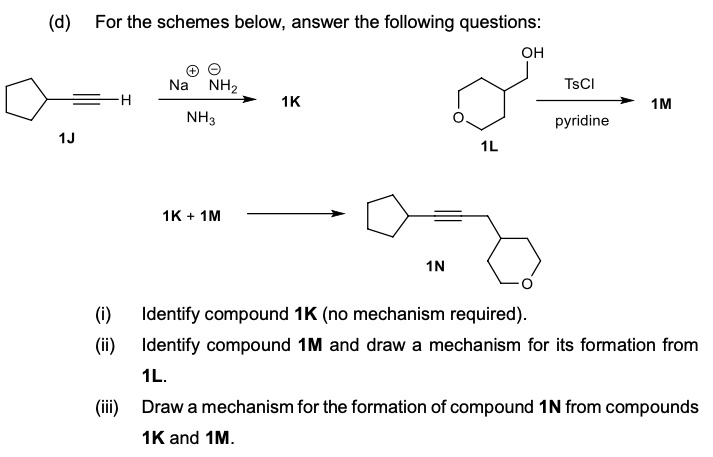
SOLVED:(d) For the schemes below; answer the following questions: OH Na NH2 NH3 TsCl ~H 1K 1M pyridine 1K 1M Identify compound 1K (no mechanism required) Identify compound 1M and draw a

organic chemistry - Is this mechanism for the formation of a tosylate correct? - Chemistry Stack Exchange

Draw the structure of the product that is formed when the compound shown below is treated with the following reagents: 1) TsCI, pyridine; 2) NaBr. Show the appropriate stereochemistry. | Study.com

organic chemistry - Why do tosylation and mesylation of alcohols follow different mechanisms? - Chemistry Stack Exchange

What is the mechanism for the following alcohol with p-TsCl/pyridine followed by addition of a strong base | Study.com
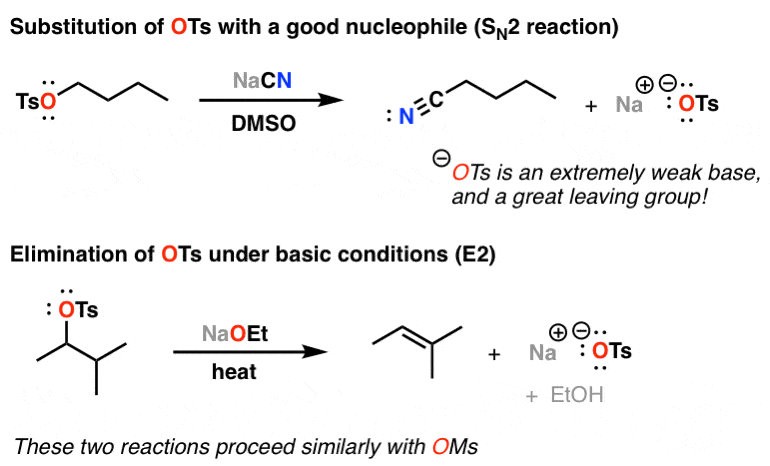
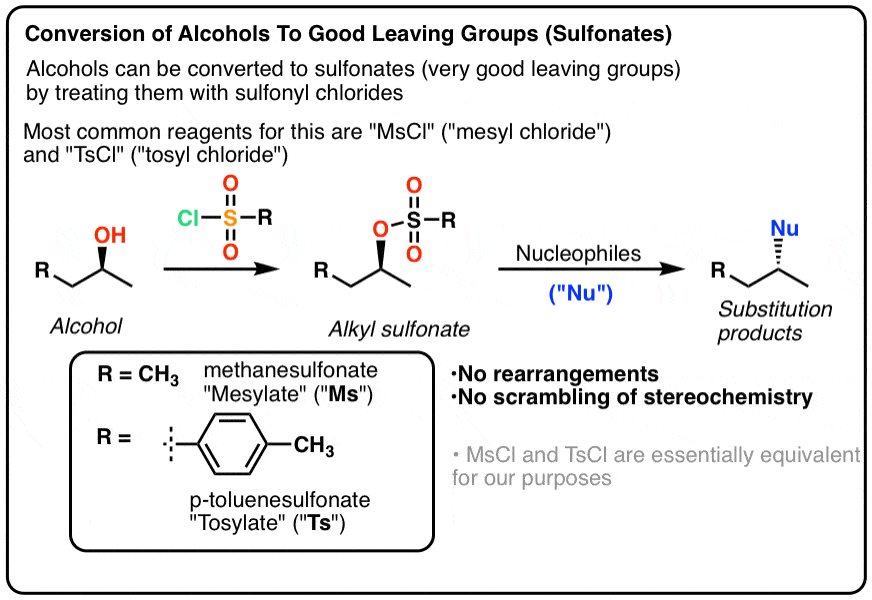
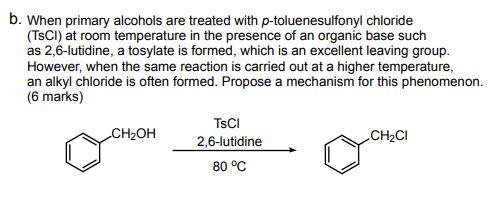
Reagent Friday: TsCl (p-toluenesulfonyl chloride) and MsCl (methanesulfonyl chloride) – Master Organic Chemistry

N-Sulfonylation of amines, imides, amides and anilides using p-TsCl in presence of atomized sodium in EtOH–THF under sonic condition - ScienceDirect

A simple synthesis of ketone from carboxylic acid using tosyl chloride as an activator - ScienceDirect
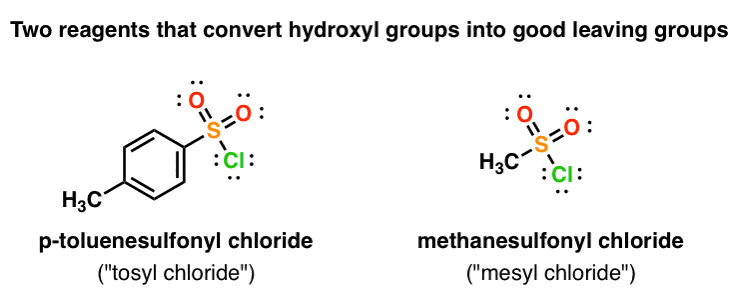
Reagent Friday: TsCl (p-toluenesulfonyl chloride) and MsCl (methanesulfonyl chloride) – Master Organic Chemistry