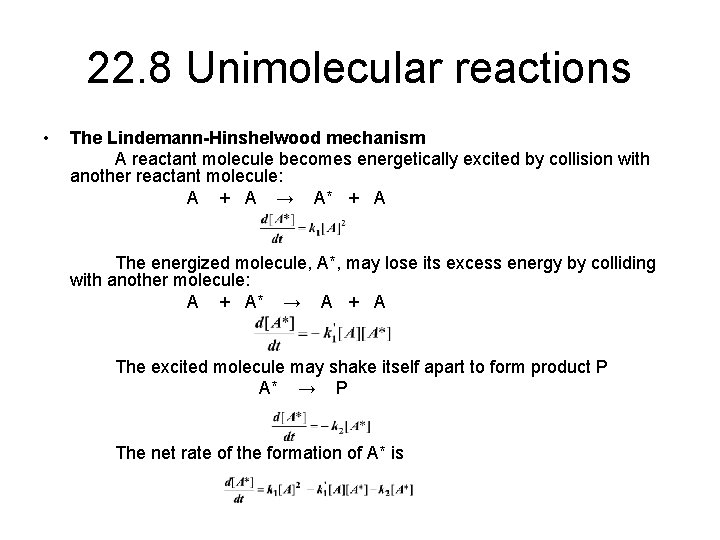
Chemistry 232 Complex Reaction Mechanisms. Lindemann-Hinshelwood Mechanism An early attempt to explain the kinetics of complex reactions. Mechanism Rate. - ppt download

How/Why have the arranged the Lindemann-Hinshelwood mechanism like this for 2 pressures (3rd line? : r/chemhelp
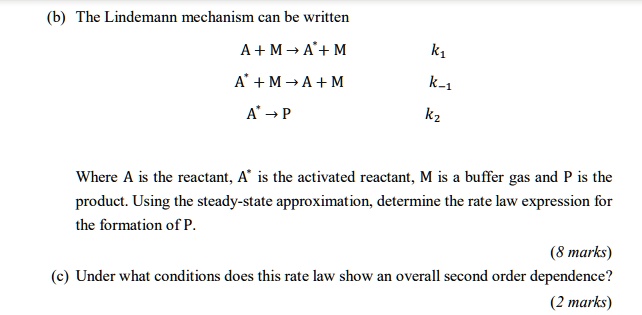
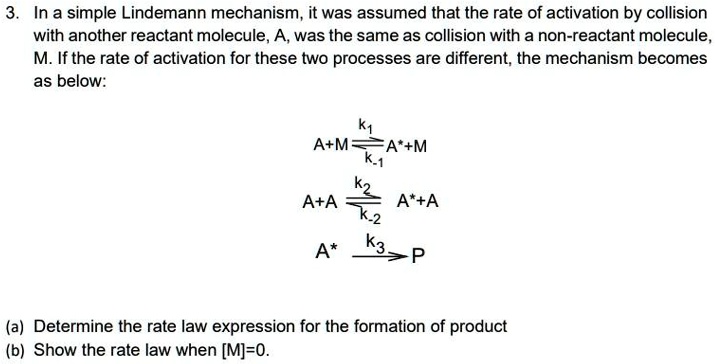
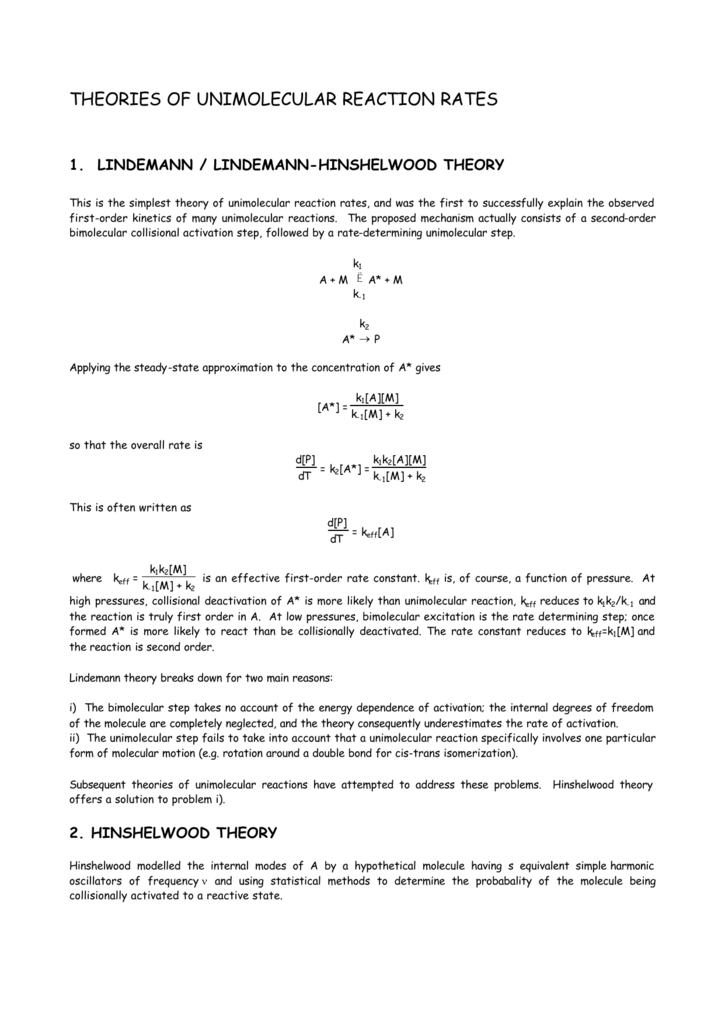
SOLVED:The Lindemann mechanism can be written A+M-A+M A +M-A+M k-1 A -P Where A is the reactant; A" is the activated reactant; M is a buffer gas and P is the product:
Solved] 16b The effective rate constant for a gaseous reaction that has a Lindemann-Hinshelwood mechanism is 1.7 x 10 3s at 1.09 kPa and 2.2 x 10 #s... | Course Hero
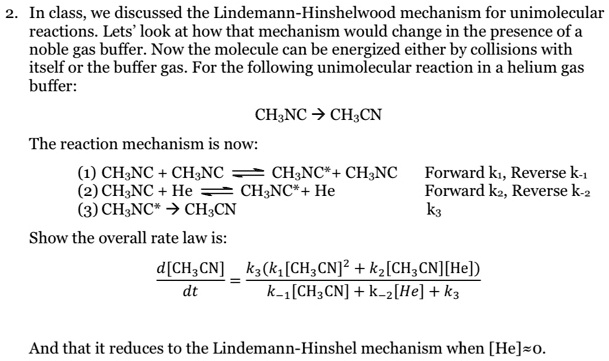
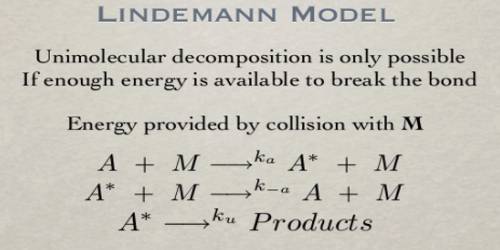
SOLVED:In class_ we discussed the Lindemann-Hinshelwood mechanism for unimolecular reactions. Lets' look at how that mechanism would change in the presence of a noble gas buffer: Now the molecule can be energized

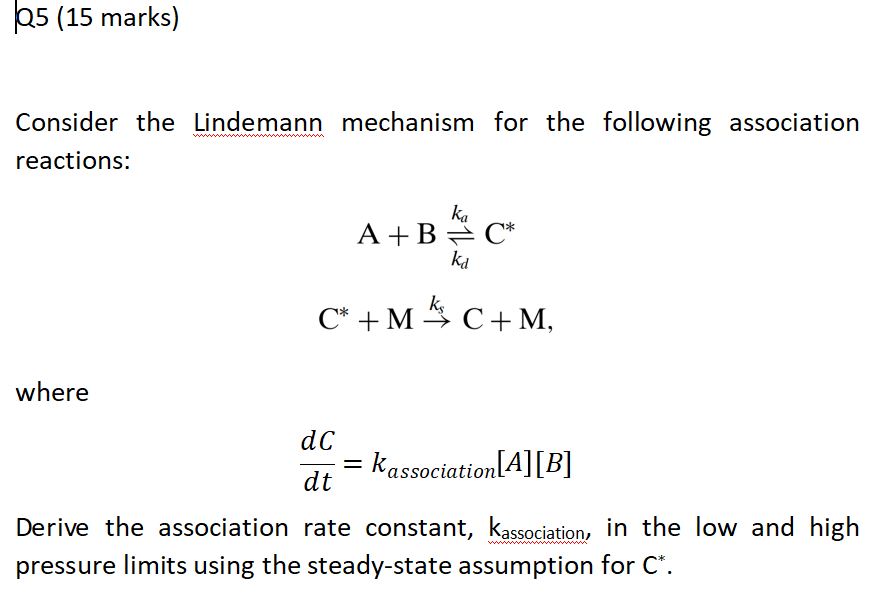
Self-test 22.8 Derive the rate law for the decomposition of ozone in the reaction 2O3(g) → 3O2(g) on the basis of the following mechanism O3 → O2 + O. - ppt video
![SOLVED:Find the rate law expression for the following proposed Lindemann mechanism ofa reactant A in the presence of an inert molecule M when the [M] = 0. A+M A* +M A +A A* + SOLVED:Find the rate law expression for the following proposed Lindemann mechanism ofa reactant A in the presence of an inert molecule M when the [M] = 0. A+M A* +M A +A A* +](https://cdn.numerade.com/ask_images/0c7779bfd1a24997b917a080935e5773.jpg)
SOLVED:Find the rate law expression for the following proposed Lindemann mechanism ofa reactant A in the presence of an inert molecule M when the [M] = 0. A+M A* +M A +A A* +

Classical Lindemann mechanism with same parameters and initial values... | Download Scientific Diagram